Medical Device Definition 201(H) . standards for medical devices. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,.
from sternmed.de
standards for medical devices. (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof.
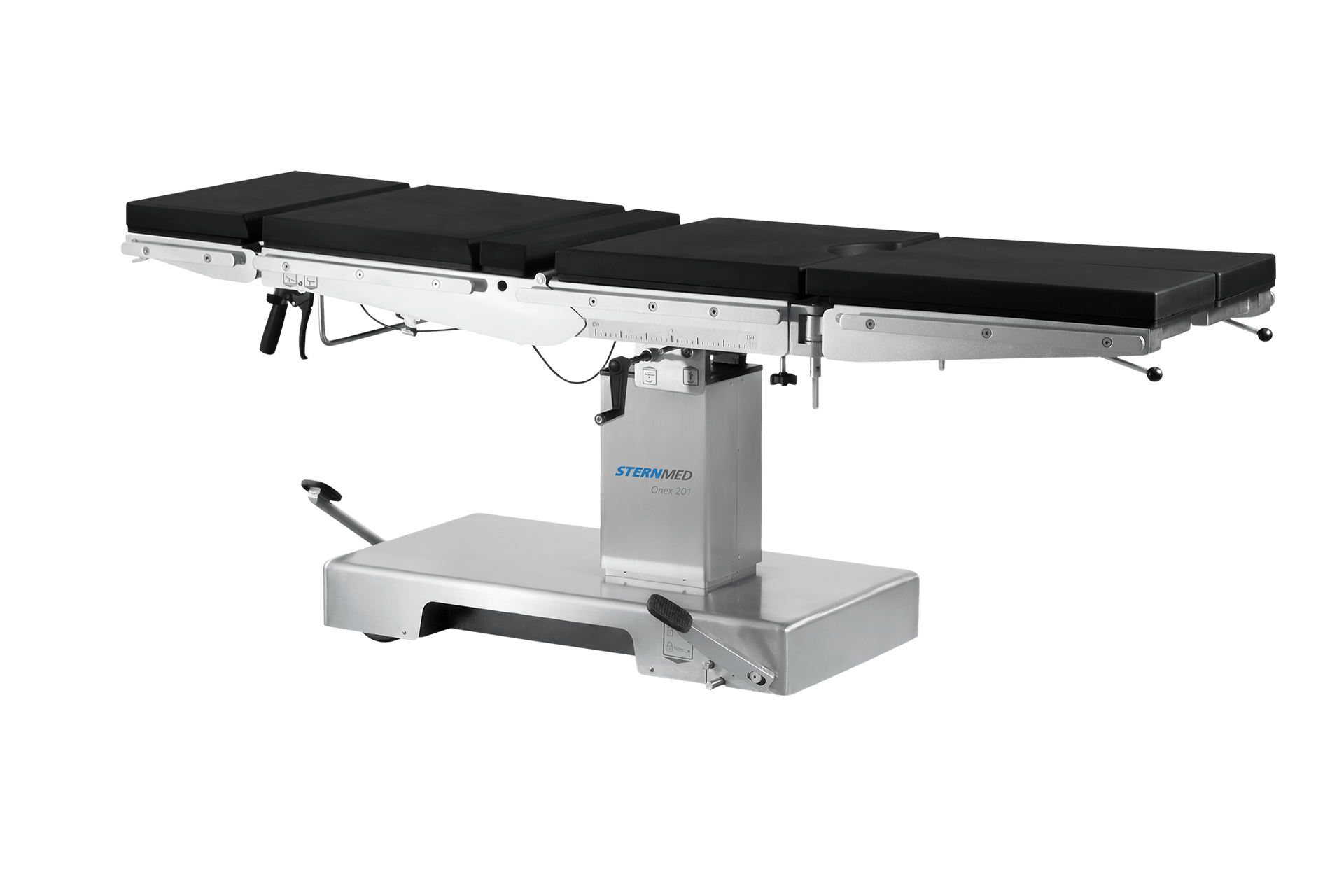
Operating table Onex 201 Medical device manufacturer SternMed
Medical Device Definition 201(H) section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: standards for medical devices. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: (n) product means any article that contains any drug as defined in section 201(g)(1) of the act;
From exocohswx.blob.core.windows.net
Type B Medical Equipment Symbol at Wesley Hamlin blog Medical Device Definition 201(H) fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. the 21st century cures act, enacted in december 2016, amended the definition of “medical. Medical Device Definition 201(H).
From www.tramremisedehallen.nl
Understanding Medical Devices Regulations to Guarantee Compliance Medical Device Definition 201(H) section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. standards for medical devices. (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; Section 201(h) of. Medical Device Definition 201(H).
From www.learngxp.com
How to Define If Your Product is a Medical Device LearnGxP Medical Device Definition 201(H) fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term. Medical Device Definition 201(H).
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Definition 201(H) however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as. Medical Device Definition 201(H).
From www.ce-marking.com
Guide on Class IIb MDD Medical Devices CE marking (mark) & European Medical Device Definition 201(H) the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. fda’s determination of whether. Medical Device Definition 201(H).
From www.youtube.com
HemoCue Hb 201+ Instructions film YouTube Medical Device Definition 201(H) (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. standards for medical devices. however, the definition of device was redesignated to section 201(h)(1) of the fd&c. Medical Device Definition 201(H).
From www.youtube.com
HemoCue® Hb 201+ System Unboxing SetUp Measure Cleaning Medical Device Definition 201(H) the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. standards for medical devices. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. fda’s determination of whether to classify a product as a drug or device is. Medical Device Definition 201(H).
From www.youtube.com
PDIS 201 H I A & CONTROL TECHNIQUES QUESTION PAPER 09 05 22 YouTube Medical Device Definition 201(H) section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: standards for medical devices. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,.. Medical Device Definition 201(H).
From sterlingmedicaldevices.com
Medical Device Engineering It Takes All Types Medical Device Definition 201(H) Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. standards for medical devices. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device”. Medical Device Definition 201(H).
From pharpoint.com
Medical Device Clinical Trials Classification & Challenges PharPoint Medical Device Definition 201(H) the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. standards for medical devices. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. the 21st century cures act, enacted in december 2016, amended the definition of “medical device”. Medical Device Definition 201(H).
From www.presentationeze.com
FDA Medical Device Labeling.PresentationEZE Medical Device Definition 201(H) Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. the fda's definition of a medical device is based on the definition found in section 201(h) of the. Medical Device Definition 201(H).
From www.qtimeconsult.com
GMP Medical Device Medical Device Definition 201(H) fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; the fda's definition of a medical device is based on the definition found in section 201(h) of the. Medical Device Definition 201(H).
From www.pinterest.es
ISO 13485 Basics and How to Get Started (QMS for Medical Devices Medical Device Definition 201(H) the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device”. Medical Device Definition 201(H).
From sternmed.de
Operating table Onex 201 Medical device manufacturer SternMed Medical Device Definition 201(H) standards for medical devices. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term. Medical Device Definition 201(H).
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Definition 201(H) however, the definition of device was redesignated to section 201(h)(1) of the fd&c act, and the new term “counterfeit device” and. the 21st century cures act, enacted in december 2016, amended the definition of “medical device” in section. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as. Medical Device Definition 201(H).
From www.slideserve.com
PPT Implantable Medical Devices PowerPoint Presentation ID2409947 Medical Device Definition 201(H) section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or component thereof. fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. standards for. Medical Device Definition 201(H).
From www.mdpi.com
Nanomaterials Free FullText Evolution of Wearable Devices with Medical Device Definition 201(H) standards for medical devices. section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: fda’s determination of whether to classify a product as a drug or device is based on statutory definitions, as set forth in. the fda's definition of a medical device is based on the definition found in section. Medical Device Definition 201(H).
From nursekey.com
Procedure Coding (HCPCS and ICD10PCS) Nurse Key Medical Device Definition 201(H) the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. (n) product means any article that contains any drug as defined in section 201(g)(1) of the act; standards for medical devices. Section 201(h) of the fd&c act defines a device as an instrument, apparatus, similar article, or. Medical Device Definition 201(H).